In the realm of physics, thermal energy holds a pivotal position, shaping our understanding of heat and temperature. ps physics chapter 6 thermal energy embarks on an illuminating journey, unraveling the intricate concepts that govern this fundamental form of energy.
From the distinction between heat and temperature to the practical applications of thermal energy, this chapter delves into the heart of the matter, providing a comprehensive exploration of a topic that touches upon various aspects of our daily lives.
Thermal Energy: Ps Physics Chapter 6 Thermal Energy

Thermal energy, also known as heat, is the energy associated with the random motion of atoms and molecules in a substance. It is a form of energy that can be transferred from one object to another through conduction, convection, or radiation.
Thermal energy is different from temperature. Temperature is a measure of the average kinetic energy of the particles in a substance, while thermal energy is the total amount of kinetic energy of all the particles in a substance.
Examples of Thermal Energy
Thermal energy is found in all forms of matter, and it is responsible for many of the phenomena that we observe in everyday life. For example, thermal energy is what causes the air to feel warm on a summer day, and it is what causes ice to melt.
Specific Heat Capacity

Specific heat capacity is a physical property that measures the amount of heat energy required to raise the temperature of a unit mass of a substance by one degree Celsius or Kelvin. It is a measure of a substance’s ability to store thermal energy.
Significance of Specific Heat Capacity
Specific heat capacity plays a crucial role in various fields, including engineering, materials science, and thermodynamics. It is essential for understanding heat transfer processes, thermal energy storage, and temperature regulation in different systems.
Variation in Specific Heat Capacity
The specific heat capacity of a substance varies significantly among different materials. Factors such as molecular structure, atomic mass, and intermolecular forces influence the specific heat capacity. Generally, substances with strong intermolecular forces, such as water, have higher specific heat capacities than substances with weak intermolecular forces, such as gases.
Table of Specific Heat Capacities
The following table provides a comparison of the specific heat capacities of common materials:
| Material | Specific Heat Capacity (J/g°C) |
|---|---|
| Water | 4.187 |
| Aluminum | 0.903 |
| Iron | 0.449 |
| Copper | 0.385 |
| Glass | 0.84 |
| Wood | 1.7 |
Heat Transfer
Heat transfer is the movement of thermal energy from one object or region to another. It occurs in three primary modes: conduction, convection, and radiation.
Conduction, Ps physics chapter 6 thermal energy
Conduction is the transfer of heat through direct contact between objects. For example, when you touch a hot stove, heat from the stove is transferred to your hand through conduction. The rate of heat transfer by conduction depends on the temperature difference between the objects, the surface area in contact, and the material of the objects.
Convection
Convection is the transfer of heat through the movement of a fluid. For example, when you boil water, heat from the bottom of the pot is transferred to the water through convection. The rate of heat transfer by convection depends on the temperature difference between the fluid and the surrounding environment, the speed of the fluid, and the density of the fluid.
Radiation
Radiation is the transfer of heat through electromagnetic waves. For example, heat from the sun reaches Earth through radiation. The rate of heat transfer by radiation depends on the temperature of the object emitting the radiation, the distance between the objects, and the nature of the intervening medium.
Calorimetry
Calorimetry is the scientific study of heat and its transfer between objects. It is a branch of thermodynamics that deals with the measurement of heat energy and the changes that occur in the thermal properties of matter.
Calorimetry experiments involve measuring the heat transferred between two objects at different temperatures. The experimental setup typically consists of a calorimeter, which is an insulated container designed to minimize heat loss to the surroundings. One object, known as the sample, is placed inside the calorimeter, and the other object, known as the heat source or heat sink, is brought into thermal contact with the sample.
Experimental Procedure
A step-by-step procedure for conducting a calorimetry experiment is as follows:
- Calibrate the calorimeter by measuring the temperature change when a known amount of heat is added to it.
- Measure the initial temperature of the sample.
- Bring the heat source or heat sink into thermal contact with the sample.
- Monitor the temperature of the sample until it reaches thermal equilibrium with the heat source or heat sink.
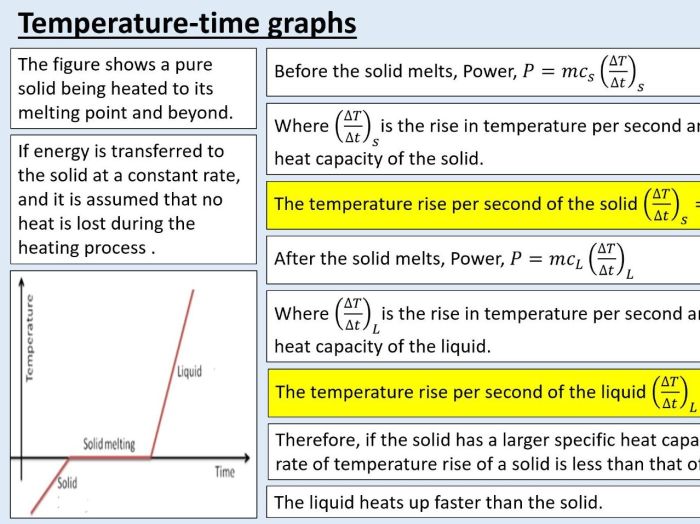
- Calculate the heat transferred between the sample and the heat source or heat sink using the equation Q = m c ΔT, where Qis the heat transferred, mis the mass of the sample, cis the specific heat capacity of the sample, and ΔTis the change in temperature.
Applications of Thermal Energy
Thermal energy has a wide range of applications across various fields, including power generation, heating, and cooling. Its versatility and efficiency make it a crucial component in modern society.
Power Generation
Thermal energy is harnessed to generate electricity in thermal power plants. Fossil fuels (e.g., coal, natural gas), nuclear fuel, or renewable sources (e.g., solar, geothermal) heat a working fluid, which expands and drives a turbine connected to a generator. This process converts thermal energy into electrical energy.
Heating and Cooling
Thermal energy is used for heating and cooling in residential, commercial, and industrial settings. Heating systems utilize furnaces, boilers, or heat pumps to distribute warm air or hot water throughout a building. Conversely, cooling systems use air conditioners or refrigerators to remove heat from a space, creating a cooler environment.
Sustainable Energy Systems
Thermal energy plays a significant role in sustainable energy systems. Solar thermal collectors harness solar energy to heat water or generate steam for power generation or heating. Geothermal energy utilizes the Earth’s natural heat to provide heating and cooling solutions.
Thermal storage systems store thermal energy during periods of excess supply for later use, optimizing energy efficiency.
Frequently Asked Questions
What is the difference between heat and temperature?
Heat is the transfer of thermal energy between objects, while temperature measures the average kinetic energy of particles within a substance.
How does specific heat capacity affect the temperature change of a substance?
Specific heat capacity measures the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius. Substances with high specific heat capacities require more heat to achieve the same temperature change.
What are the three modes of heat transfer?
Conduction, convection, and radiation are the three primary modes of heat transfer.